Parkinson’s Drug Alters Microbiome, Triggers Iron Deficiency – Neuroscience News
Summary: The Parkinson’s drug entacapone disrupts the gut microbiome by causing iron deficiency, helping the growth of harmful bacteria such as E. coli. Using advanced molecular techniques, the researchers found that entacapone interferes with the availability of iron, an important resource for many intestinal parasites.
This pattern of gut dysbiosis highlights the broad implications of how human-targeted drugs affect microbial life. Reducing these effects, such as repletion of iron in a selective way, can help reduce side effects and support better treatment outcomes.
This work highlights the complex relationship between drugs, the gut microbiome, and overall health. The findings pave the way for future microbiome-targeted drug designs.
Important information:
- Entacapone destroys the gut microbiome by causing iron deficiency, which favors bacteria such as E. coli which thrive under low iron conditions.
- Advanced techniques, including bulk water labeling and Raman spectroscopy, have revealed subtle changes in biological activity that span multiple scales.
- Ensuring a targeted iron supply to the gut can reduce drug-induced dysbiosis while maintaining therapeutic efficacy.
Source: University of Vienna
In a new basic research, carried out within the framework of the “Cluster of Excellence” funded by the FWF “Microbiomes drive Planetary Health”, scientists from the University of Vienna, in collaboration with the University of Southampton, Aalborg University and Boston University, revealed that the most prescribed drug for Parkinson’s disease entacapone it severely damages the human gut microbiome by causing iron deficiency.
Study, published in Nature Microbiology, provides new insights into the often overlooked impact of human-targeted drugs on the microbial communities that play an important role in human health.
While it’s clear that antibiotics can seriously damage a person’s microbiome, emerging research shows that many types of drugs targeted at humans—especially those used to treat neurological conditions—can also affect by sinking the communities of microbes that live in our bodies.
Although there are effects intended to treat different organs, these drugs can intentionally disrupt the balance of intestinal microbes, which leads to possible health consequences.
Until now, most studies investigating these interactions have relied on patient cohort analyzes affected by many confounding factors or on experiments using specific gut bacteria, which do not fully understand the complexity of the microbiome. of a person.
A Novel Study Design for Investigating Drug-Bug Interactions
Using a new experimental method, an international team studied the effects of two drugs—entacapone and loxapinea schizophrenia drug—in stool samples from healthy people.
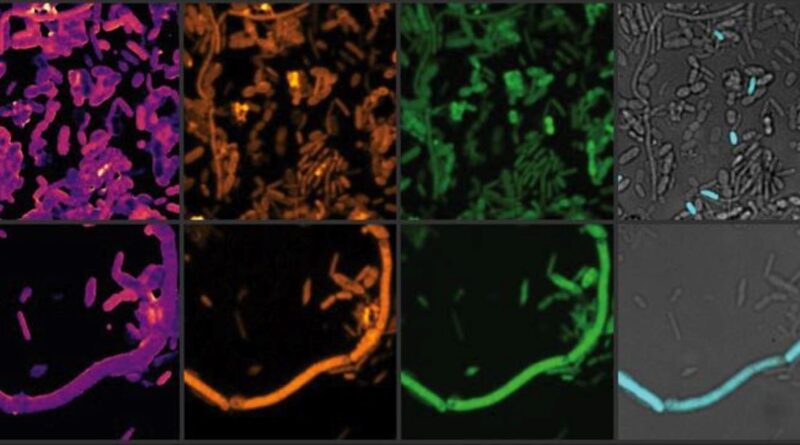
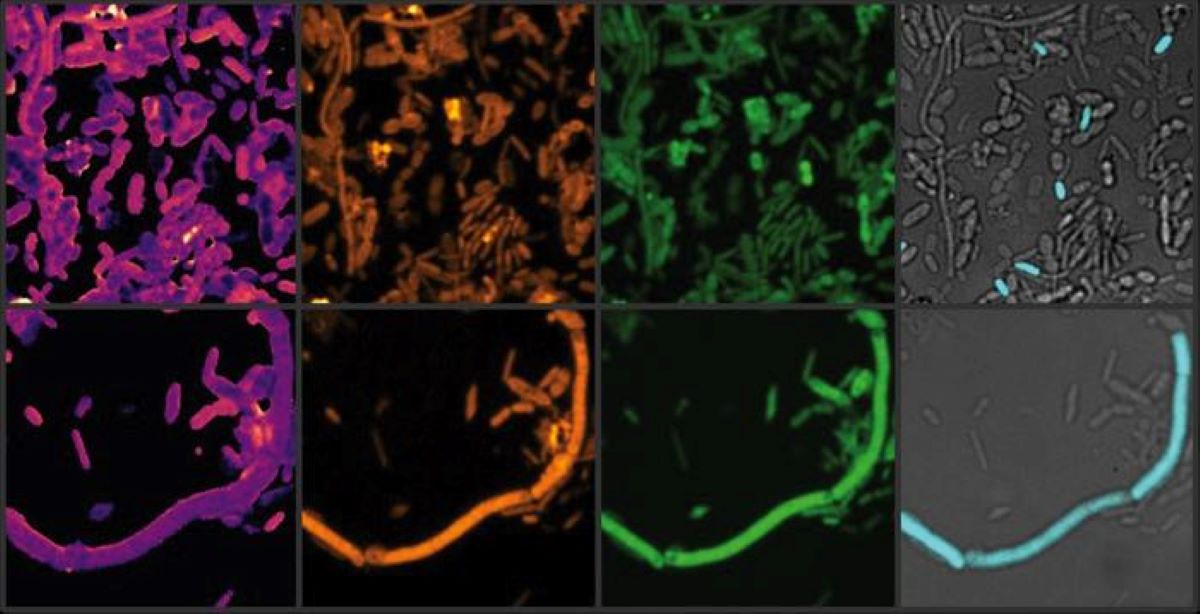
They infused samples with therapeutic components of these drugs, then analyzed the impact on microbial communities using advanced molecular and imaging techniques, including heavy water labeling combined with Stimulated Raman Spectroscopy (SRS).
The team found that loxapine and more than entacapone inhibited many members of the microbiome, while E. coli is greatly enhanced in the presence of entacapone.
“The results were even more impressive when we analyzed the activity of the organisms, rather than their abundance,” explained Fatima Pereira, lead author of the study and former Postdoctoral researcher at the University of Vienna .
“The water-heavy SRS method allowed us to detect subtle but important changes in the gut microbiome, which are often missed by traditional mass-based measurements.”
Entacapone Induces Iron Starvation, Favors Pathogenic Bacteria
Researchers speculate that entacapone may interfere with the availability of iron in the gut, which is an important source for many pathogens. Their experiments confirmed that adding iron to stool samples containing entacapone counteracted the drug’s mutagenic effects.
Further research revealed that E. colithat thrived under these conditions, had a very good iron uptake system (enterobactin siderophore). This system allowed the bacteria to overcome iron starvation and proliferate, even in the presence of the drug.
“By showing that entacapone causes iron deficiency, we have discovered a new mechanism of drug-induced dysbiosis, which drugs selectively target. E. coli and other microorganisms that may have organisms that are well adapted to iron reduction conditions,” said Michael Wagner, scientific director of the Excellence Cluster and deputy head of the Center for Microbiology and the Environment Systems Science (CeMESS) at the University of Vienna.
Broader Implications for Drug-Microbiome Interactions
The discovery has broader implications for understanding how other targeted drugs in humans may affect the microbiome. Several drugs, including entacapone, have metal-binding catechol groups, suggesting that this mechanism may be a common pathway for drug-induced microbiome changes.
Studies also offer the possibility of reducing the side effects of drugs such as entacapone. By ensuring that there is enough iron in the large intestine, it is possible to reduce dysbiosis and intestinal problems that often accompany the treatment of Parkinson’s disease.
“The next step is to explore how we can modify drug therapies to support the gut microbiome,” Wagner said.
“We are looking at strategies to selectively deliver iron to the large intestine, where it can enrich the microbiome without interfering with drug absorption in the small intestine.”
About Education: This study was carried out as part of the Group of Excellence funded by the FWF “Microbiomes drive Planetary Health,” a collaborative research project including eight Austrian Research Institutes.
About this research issues neuropharmacology and the microbiome
Author: Alexandra Frey
Source: University of Vienna
Contact: Alexandra Frey – University of Vienna
Image: Image courtesy of Xiaowei Ge (Boston University)
Basic research: Open access.
“The Parkinson’s drug entacapone disrupts gut microbiome homeostasis through iron uptake” by Michael Wagner et al. Nature Microbiology
Summary
The Parkinson’s disease drug entacapone disrupts gut microbiome homeostasis through iron uptake.
Many human-targeted drugs alter the gut microbiome, leading to adverse effects on the host’s health. However, the mechanisms underlying these effects are not well understood.
Here we combined quantitative microbiome profiling, long-term readout metagenomics, stable isotope analysis and single-cell chemical imaging to investigate the effect of two widely prescribed drugs on the microbiome of stomach
Physiological levels of entacapone, a treatment for Parkinson’s disease, or loxapine succinate, used to treat schizophrenia, were measured ex vivo in human stool samples.
Both drugs affect bacterial activity significantly, more than bacterial abundance. Mechanistically, entacapone can complex and destroy existing iron causing microbiome composition and functional changes. Microbial growth can be saved by replenishing the available iron levels of the microbiota.
In addition, entacapone-induced iron starvation selected for iron microbiome members that induce antimicrobial resistance and genes.
These findings reveal the effect of two under-researched drugs on whole microbiomes and point to iron diversion as a way to interfere with drug-induced pathogens.
#Parkinsons #Drug #Alters #Microbiome #Triggers #Iron #Deficiency #Neuroscience #News